I Aniline is an aromatic amine in which the group is directly attached to. Complete step by step answer.
Draw All The Possible Resonance Structures Of Benz Class 12 Chemistry Cbse
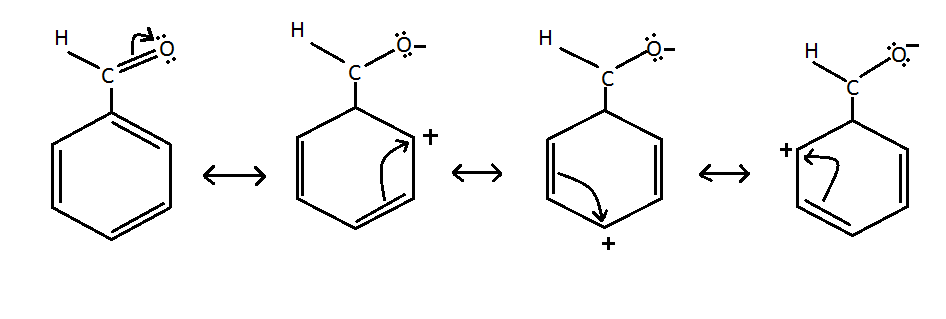
The resonating structures of benzaldehyde are represented as.

. What are examples of electron releasing and electron. E C 6 H 5 CH 2. Add your answer and earn points.
The number of resonance structures varies depending upon the number of electrons it has. Advertisement Remove all ads. An aromatic amine such as aniline exhibits a resonance effect due to.
The resonating structures of benzaldehyde are represented as. Draw the resonance structures of benzaldehyde Brainly in Form zero formal charges most significant pin them create your own moodboard positive located either ortho partha positions only electrophilic substitution. Draw the resonance structure for the following compound.
Draw correct structure for benzaldehyde. I Aniline is an aromatic amine in which the group is directly attached to hybridized carbon of benzene ring. Join Login Class 11.
It has a role as a flavouring agent a fragrance an odorant receptor agonist a plant metabolite an EC 3551 nitrilase inhibitor and an EC 3113 triacylglycerol lipase inhibitor. Draw resonance structures of the following. Because three of them place a positive charge on a carbon atom of the benzene ring a CHO group withdraws electron density from a benzene ring by a resonance effect.
Advertisement Remove all ads. Add curved arrows to the resonance structures for benzaldehyde to illustrate the electron-withdrawing groups effect on the aromatic ring. The structures contain a negative charge on oxygen and positive charge on the carbon present in the benzene ring.
Concept Notes Videos 423. It is basically an aldehyde group substituted with one of the hydrogen atoms of benzene rings. Draw a resonance structure of the following.
Select Draw Rings More Erase 4 By observing the resonance structures at what positions will. Maharashtra State Board HSC Science General 11th. Group is an electron donating group.
Benzaldehyde Cas 17901 93 8 Chemsrc Name reaction of organic chemistry required expertise in the above N. C 6 H 5 CH 2. It is basically an aldehyde group substituted with one of the hydrogen atoms of benzene rings.
Sci 2022 13 3915 DOI. The resonance structures of benzaldehyde c 6. Draw the resonating structure of Aniline.
The resonance contributors for the chlorobenzene. Question Bank Solutions 5546. Draw the resonance structures of benzaldehyde.
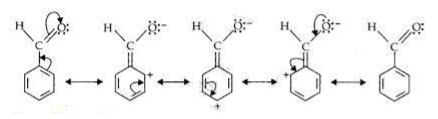
Draw all the no-bond resonance structures of isopropyl carbocation. The resonating structures of benzaldehyde are. For example seven resonance structures can be drawn for benzaldehyde C HsCHO.
How many resonance structures can be drawn for N_2O. There are in total four resonance structures. Structure and Classification of Amines.
The cyclic structure of benzene molecules is made up of alternating. Resonance is the concept where electrons bonds are delocalized over three or more atoms which cannot be depicted with one simple Lewis structure. Advertisement Remove all ads.
Complete step by step answer. Draw all the possible resonance structur. The simplest aromatic aldehyde and parent of the class of benzaldehydes.
1 See answer rdharshinii30 is waiting for your help. Rdharshinii30 rdharshinii30 29102017 Chemistry Secondary School answered expert verified Draw the resonance structures of benzaldehyde. Asked Jan 4 in Chemistry by Ayushsingh 259k points basic principles of organic chemistry.
Draw the resonance structures of. Draw a resonance structure of the following. This product C6H5-NO2 is windly.
H H 3 add curved arrows to structure C to form structure D. It is basically an aldehyde group substituted with one of the hydrogen atoms of benzene rings. Resonance structures of benzaldehyde are formed due to the electronegativity of oxygen and the delocalised electrons of benzene ring.
Draw a resonance structure of the following. Benzaldehyde is an arenecarbaldehyde that consists of benzene bearing a single formyl substituent. Experts are tested by Chegg as specialists in their subject area.
What is the difference between resonance and chemical equilibrium. The number of resonance structures varies depending upon the number of electrons it has. Now if we look at benzaldehyde the molecular formula of benzaldehyde is textC_text6textH_text5textCHO.
Draw all the possible resonance structures for Benzaldehyde. How many resonance structures can be drawn for ozone. Is this an orthopara or meta director.
Draw all the resonance structure for benzaldehyde. Now if we look at benzaldehyde the molecular formula of benzaldehyde is textC_text6textH_text5textCHO. Resonance structures of benzaldehyde are formed due to the electronegativity of oxygen and the delocalised electrons of benzene ring.
D The structure of C 6 H 5 CHO is. The four resonance structures are shown. Benzaldehyde is a combination of c h and o.
Get the answers you need now. Draw a resonance structure of the following. Resonance structures of benzaldehyde are formed due to the electronegativity of oxygen and the delocalised electrons of benzene ring.
Draw all secondary resonance structures for benzaldehyde. We review their content and use your feedback to keep the quality high. Draw all the resonance structure for benzaldehyde.
What is the M and -M effect. I Aniline is an aromatic amine in which the group is directly attached to.

Draw All The Possible Resonance Structures For Benzaldehyde

Draw The Resonance Structure For Benzaldehyde Brainly In

Answered Draw The Resonance Structures Of I Phenol Ii Benzaldehyde Iii Aniline Brainly In

Draw A Resonance Structure Of The Following Benzaldehyde Chemistry Shaalaa Com

Draw The Resonating Structure Of Benzaldehyde Scholr

Super Trick Resonance Structures Of Benzaldehyde Resonance In Benzaldehyde Youtube
Draw All Possible Resonating Structures Of Benzal Class 11 Chemistry Cbse
0 komentar
Posting Komentar